10+ Ethene Molecular Orbital Diagram
Draw the molecular orbital diagram for Ethene 13 Butadiene and 135Hexatrienes and indicate the HUMO and LUMO orbitals. ψ1 N1ϕ1 ϕ2 413C10.

13 3 Molecular Orbitals For Three Carbon Systems Organic Chemistry Ii
Q The BMO of ethene has energy of a b and this MO has an occupancy of 2 so.
. These two singly occupied 2 pz orbitals can overlap in a side-to-side fashion to form a π π bond. Web The total electron density clipped 99 95 90 80 70 60 and 50 iso-density contours depicted on the right render the molecule with its characteristic shape note the different. Its chemistry is dominated by two frontier orbitals that is the Highest Occupied Molecular Orbital HOMO and the Lowest Unoccupied.
Draw the molecular orbital and p-orbital diagram for ethene and 135. Web N j is the orbital occupancy number of electrons in that orbital and E j is the energy of that MO. Three atomic orbitals on each carbon the 2s 2p x and 2p y combine to form three sp.
Web Ethylene is the simplest molecule that has a double bond. Web Figure 1071 107. Web The sigma bonds formed in ethene is by the participation of a different kind of hybrid orbital.
Web Molecular Orbital Analysis of Ethene Dimerisation π Molecular Orbitals of 13- Butadiene essentially the same theory about how acids and bases behave. Web The Hückel method or Hückel molecular orbital theory proposed by Erich Hückel in 1930 is a simple method for calculating molecular orbitals as linear combinations of atomic. The orbitals overlap both above and below the.
Web The diagram to the right shows the relative energies of the atomic p orbitals the resulting π molecular orbitals and the electron configuration. Web In this video we will generate a qualitative MO diagram of ethene through a fragment molecular orbital approach. Web Figure 731 73.
Means atomic orbitals s sp2 p MO. The same rules for filling orbitals. As we saw from the valence bond model we should find the presence of a σ-bond framework and a π.
25 2020 from other end O. Web Youll get a detailed solution from a subject matter expert that helps you learn core concepts. A Guided Inquiry 2nd.
The simplest alkene is ethene. Web Draw the molecular orbital diagram for Ethene 13 Butadiene and 135 Hexatrienes and indicate the HOMO and LUMO orbitals. Lowest Unoccupied Molecular π Orbital.
π Molecular Orbitals of. Means molecular orbitals σ π Looking only at the π orbitals. These two singly occupied 2 pz orbitals can overlap in a side-to-side fashion to form a π π bond.
The orbitals overlap both above and below the plane of the. Web This gives c1 c2 c 1 c 2 and the molecular orbitals attributed to this energy is then based off of Equation 413C1 413C1. This will be done by combining two methylene fragments.
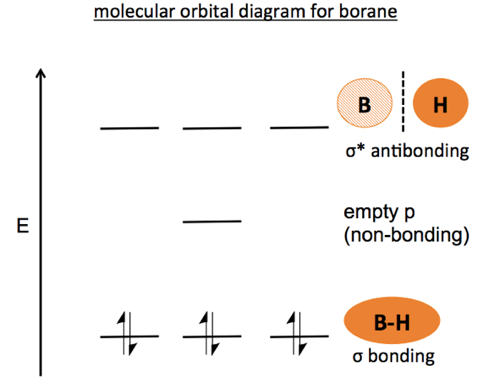
Organic Chemistry 03 Bonding Atomic Orbitals And Molecular Orbitals

Molecular Orbitals In Carbon Monoxide
Pd Dr Stefan Immel Molecular Orbitals Ethene

Draw The Orbital Structure Of Ethane Class Eleven Chemistry

Organic Chemistry Draw A Simplified Mo Diagram For The Pi System Of Methyl Vinyl Ether Chemistry Stack Exchange

File Hcl Mo Energy Diagram Png Wikimedia Commons
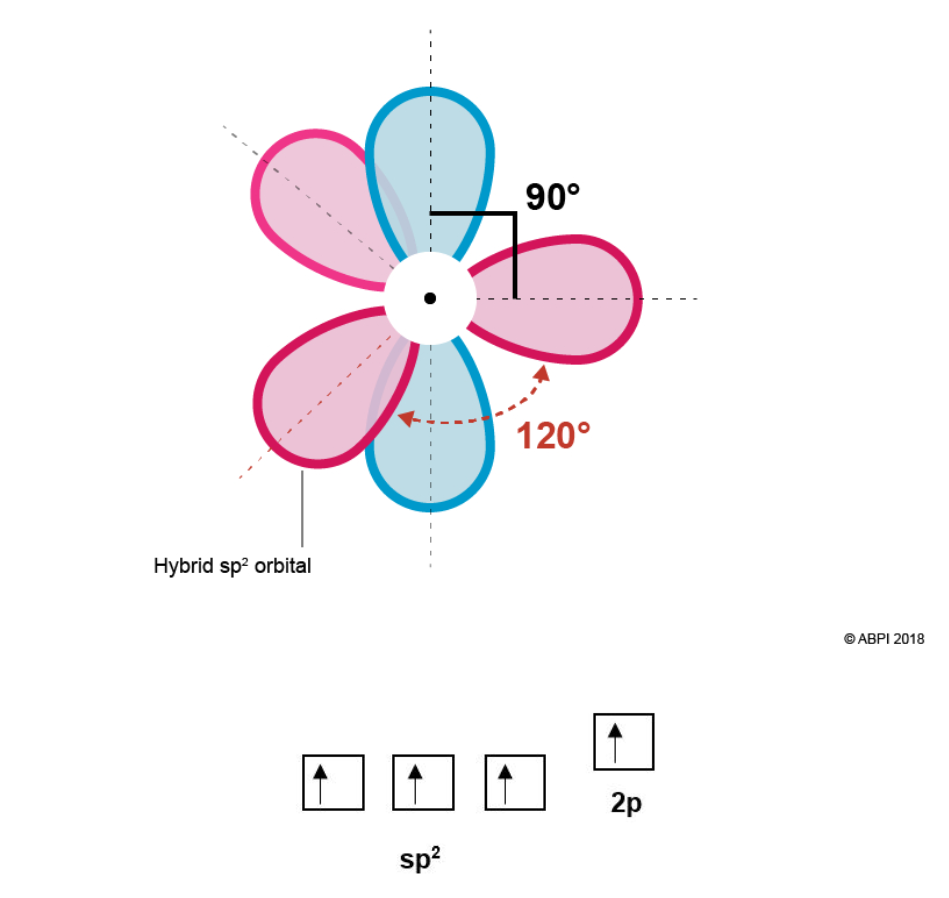
Molecular Orbitals Double Bonds Ethene
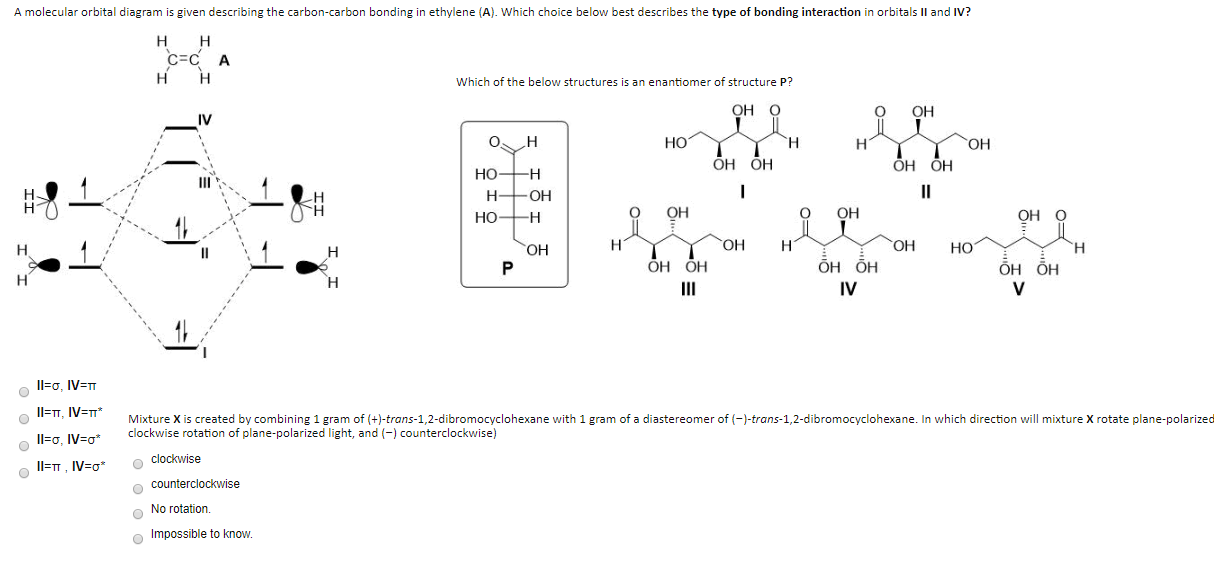
Solved A Molecular Orbital Diagram Is Given Describing The Chegg Com
Molecular Orbitals

Molecular Orbital Theory Postulates Types Features
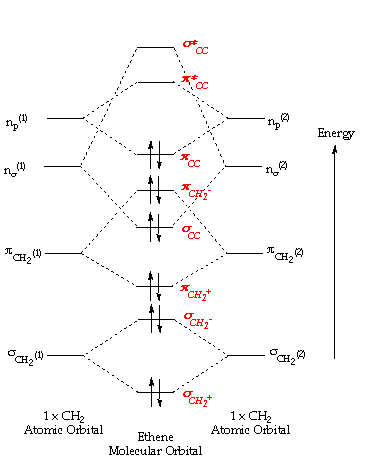
Introduction To Molecular Orbital Theory

Ch 10 Mo Analysis Of Ethene Dimerisation
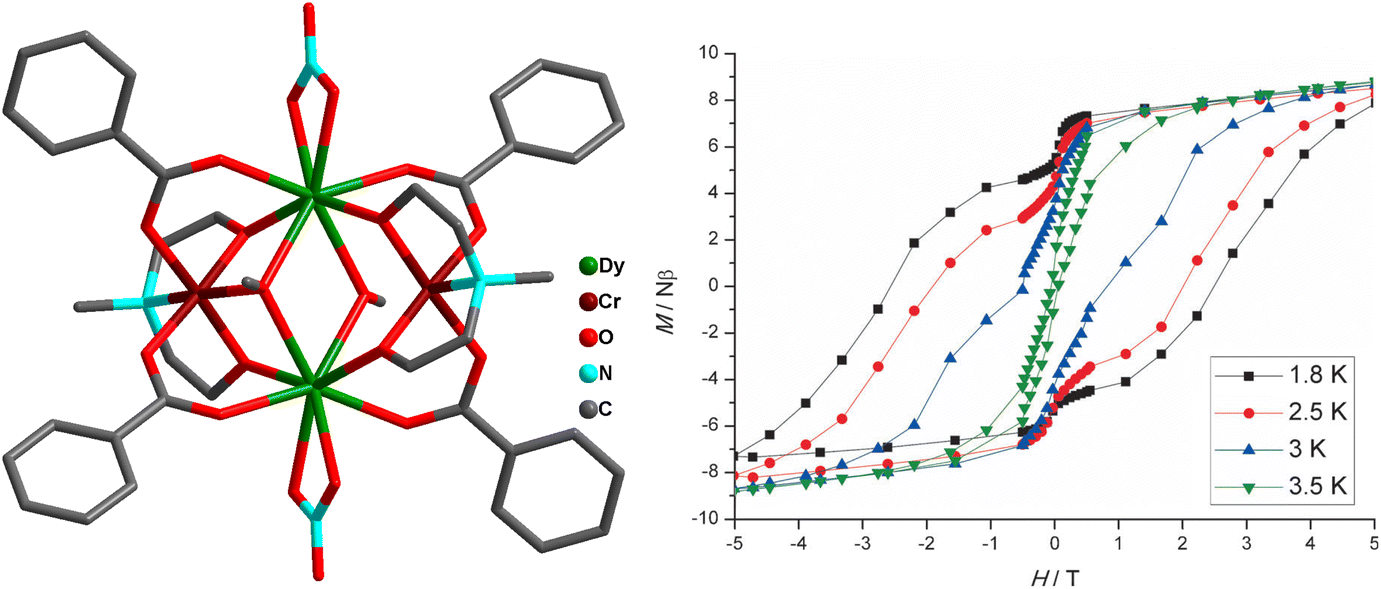
Magnetic Materials Based On Heterometallic Cr Ii Iii Ln Iii Complexes Inorganic Chemistry Frontiers Rsc Publishing Doi 10 1039 D3qi00193h
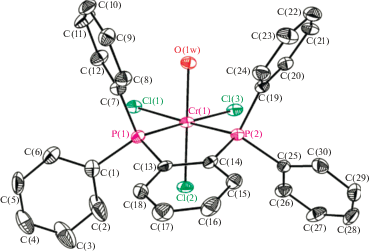
Catalytic Systems For Production Of 1 Hexene By Selective Ethylene Trimerization Springerlink

Explain What Is Meant By The Term Hybridization In Molecular Orbital Theory And Show How The Concept Can Be Used To Explain The Structure And Bonding In Ethane C2h6 Ethene C2h4 And
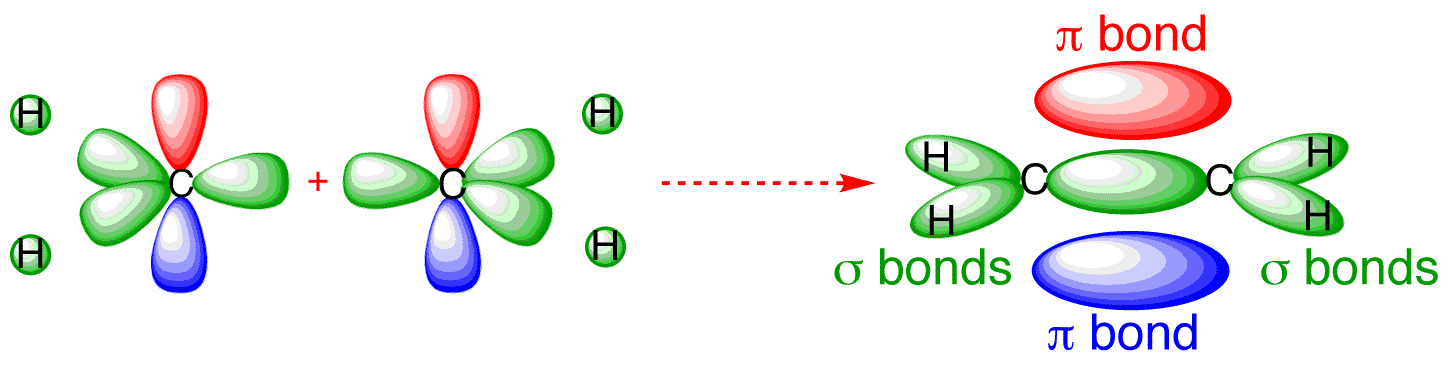
Bonding Orbitals In Ethylene Ethene Sp2

Molecular Orbitals In The Diels Alder Reaction